Products / Cleanrooms
QleanAir QleanSpace FDA cGMP
An adaptable and reliable cleanroom solution for high risk applications
QleanSpace FDA cGMP is our customizable cleanroom solution, developed to help meet FDA cGMP classification requirements. The cleanroom uses individual HEPA 14 fan filter units and airlocks to generate positive or negative pressure, allowing for a highly controlled environment. The monitoring system surveils airborne particle concentration, relative humidity, differential pressure, and temperature. Thanks to QleanSpace FDA cGMP being a freestanding and independent room-within-a-room solution, it can be installed quickly almost anywhere and is highly adaptable. Consultation, design, installation, service and functional guarantee are all included in our hassle-free cleanroom solutions.

Key components of the QleanSpace FDA cGMP solution
- Customizable, modular design, tailored for your needs in any size
- Non-porous and flush surfaces for easy cleaning
- Continuous monitoring of data points of interest, such as particle levels, relative humidity, differential pressure, and temperature, with warnings at pre-set levels
- Individual fan filter units with HEPA 14 filters certified according to EN 1822
- Fully automated and interlocking door system
- Airlocks for clean passage of personnel, pass-through cabinets for material, laminar airflow cabinets and other options available
- Including installation, preventative maintenance, and service
Benefits
Thanks to QleanSpace FDA cGMP being both modular and compact while also working working adjacent to the facility’s built-in ventilation, the cleanroom solution can be placed almost anywhere. QleanSpace FDA cGMP constantly monitors the cleanroom’s pressure levels, relative humidity, temperature, and the level of airborne particles, while displaying the information on screens visible to staff both inside and outside the area. Although the QleanSpace FDA cGMP cleanroom has rigid and durable walls that simulate the property of a permanent fixed-wall room, it is still a flexible solution that can be re-designed or expanded if needed. We help our customer with everything from needs analysis and design to installation and service. Regular maintenance, inspection of filter capacity, and performance checks are also included.
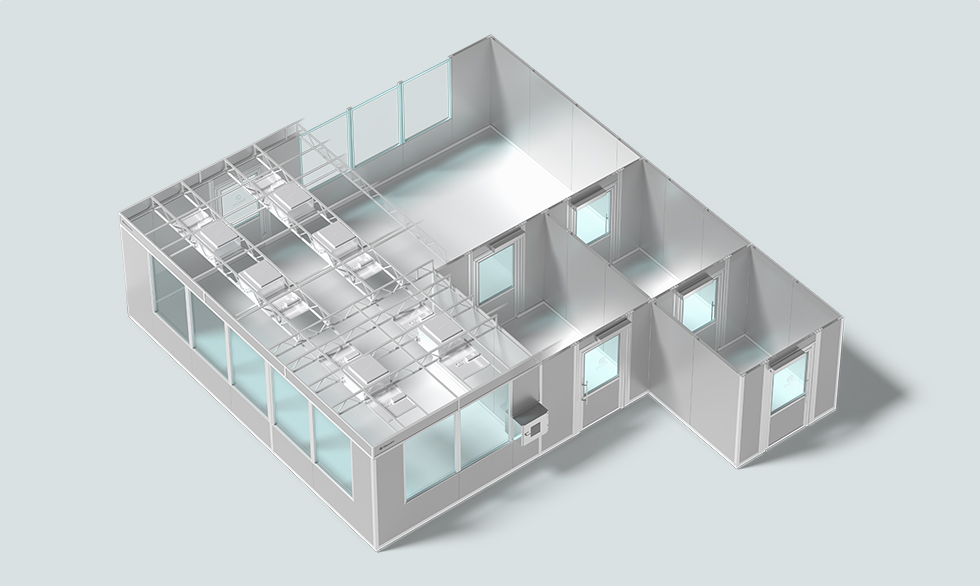
Example layout
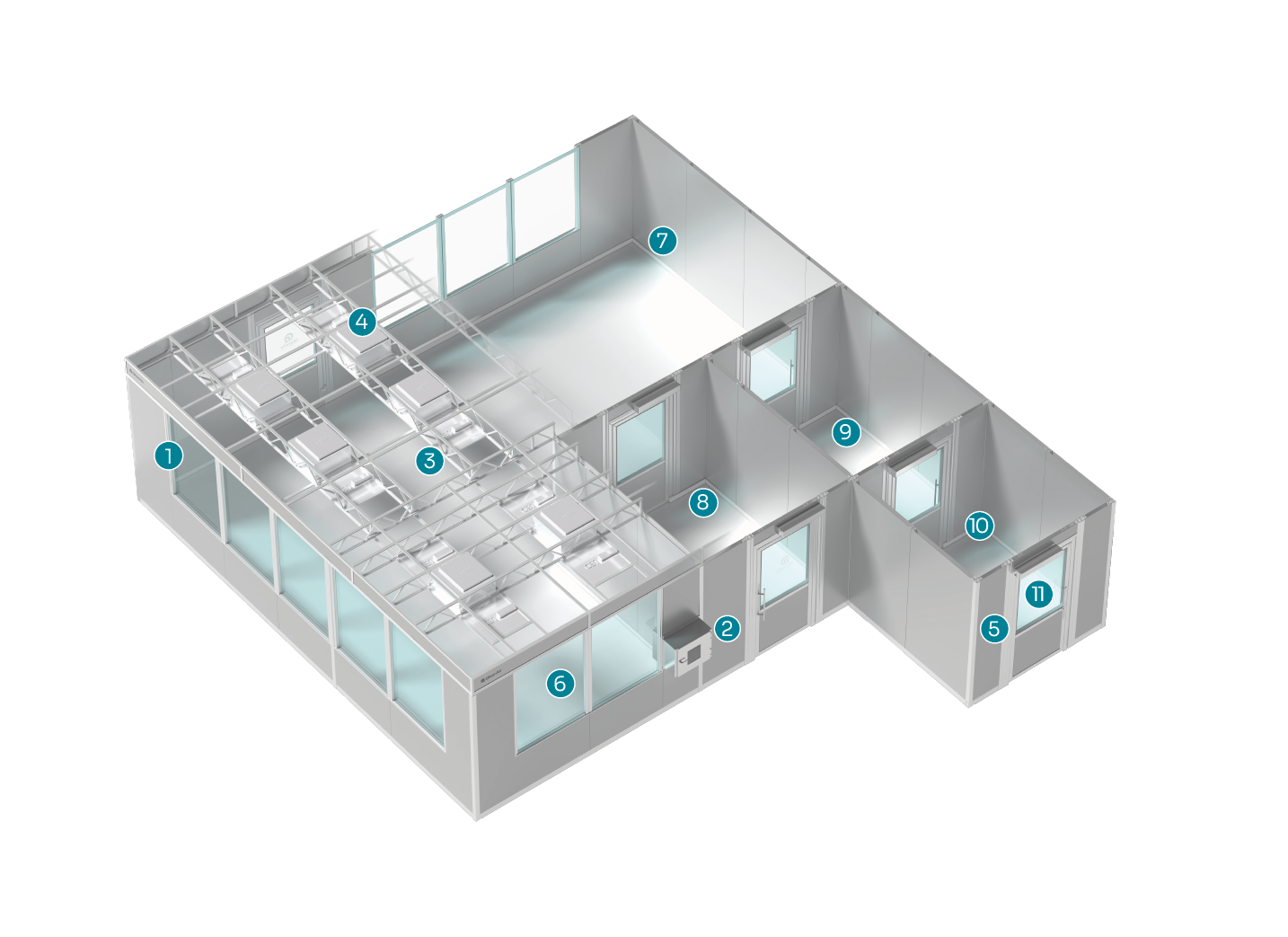
Example layout
-
Modular, durable walls
-
Pass-through cabinet
-
LED lighting
-
Fan filter units/HEPA 14 filters
-
Continuous monitoring
-
Windows/glass panels
-
Smooth surfaces and rounded corners
-
Material air lock
-
Anteroom
-
Changing room
-
Door automation